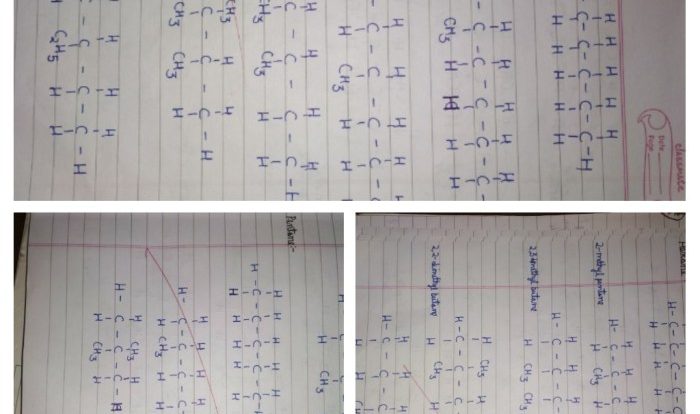
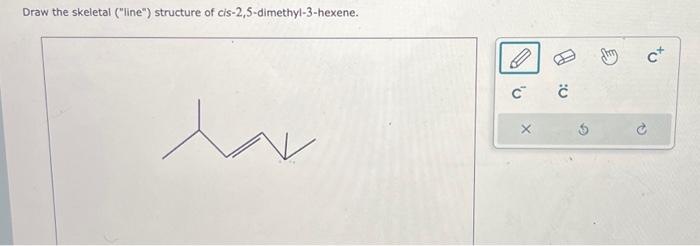
Cis 2 5 dimethyl 3 hexene – Embark on a scientific odyssey with cis-2,5-dimethyl-3-hexene, an intriguing organic compound that captivates the world of chemistry. Its unique molecular structure and versatile properties make it a subject of immense interest, inviting us to delve into its fascinating realm.
From its physical and chemical characteristics to its reactivity and spectroscopic analysis, we unravel the complexities of cis-2,5-dimethyl-3-hexene, shedding light on its diverse applications and the safety measures required for its handling. Prepare to be enthralled as we explore the intricacies of this remarkable compound.
Physical and Chemical Properties
Cis-2,5-dimethyl-3-hexene is a branched-chain alkene with the molecular formula C8HIt is a colorless liquid with a characteristic odor. The physical properties of cis-2,5-dimethyl-3-hexene are as follows:* Boiling point: 120-122 °C
Melting point
90 °C
Density
0.74 g/cm³
Refractive index
1.42
Cis-2,5-dimethyl-3-hexene is a reactive compound that can undergo a variety of chemical reactions. These reactions include:* Addition reactions: Cis-2,5-dimethyl-3-hexene can undergo addition reactions with a variety of reagents, including hydrogen, halogens, and water.
Oxidation reactions
Cis-2,5-dimethyl-3-hexene can undergo oxidation reactions with a variety of reagents, including oxygen, ozone, and potassium permanganate.
Polymerization reactions
Cis-2,5-dimethyl-3-hexene can undergo polymerization reactions to form polymers.The chemical properties of cis-2,5-dimethyl-3-hexene are due to the presence of the double bond in the molecule. The double bond is a reactive site that can undergo a variety of reactions.
Molecular Structure and Bonding
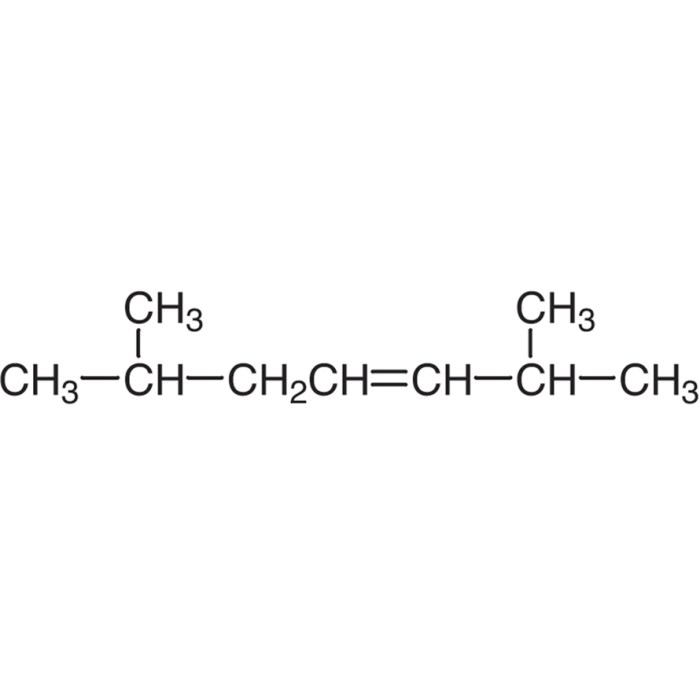
Cis-2,5-dimethyl-3-hexene has a molecular structure that consists of a carbon chain with a double bond between the third and fourth carbon atoms. The two methyl groups are attached to the second and fifth carbon atoms. The molecular structure of cis-2,5-dimethyl-3-hexene is shown below:“`CH3-CH(CH3)-CH=CH-CH(CH3)-CH2-CH3“`The double bond in cis-2,5-dimethyl-3-hexene is a pi bond.
Cis 2 5 dimethyl 3 hexene is a branched alkene with a molecular formula of C8H16. The “cis” prefix indicates that the two methyl groups on the double bond are on the same side of the molecule. The “2 5” indicates that the double bond is between the second and third carbon atoms, and the “3” indicates that the methyl group is on the third carbon atom.
The “-ene” suffix indicates that the molecule contains a double bond. To learn more about the etymology of chemical terms, check out the meaning of the root amo . Cis 2 5 dimethyl 3 hexene is a colorless liquid with a boiling point of 121-122 °C.
A pi bond is a covalent bond that is formed by the overlap of two p orbitals. The p orbitals of the two carbon atoms that are involved in the double bond are perpendicular to each other. The pi bond is weaker than a sigma bond, which is a covalent bond that is formed by the overlap of two s orbitals.The
double bond in cis-2,5-dimethyl-3-hexene is also a cis double bond. A cis double bond is a double bond in which the two substituents on the same side of the double bond. In cis-2,5-dimethyl-3-hexene, the two methyl groups are on the same side of the double bond.The
molecular structure of cis-2,5-dimethyl-3-hexene has a significant impact on its physical and chemical properties. The double bond makes the molecule more reactive than a molecule with a single bond. The cis configuration of the double bond makes the molecule more polar than a molecule with a trans configuration.
Reactivity and Reactions: Cis 2 5 Dimethyl 3 Hexene
Cis-2,5-dimethyl-3-hexene, as an alkene, exhibits high reactivity due to the presence of a double bond between the second and third carbon atoms. This double bond makes it susceptible to various chemical reactions, including addition, substitution, and elimination reactions.
Addition Reactions
In addition reactions, a molecule adds to the double bond, resulting in the formation of a new bond between each of the two carbon atoms in the double bond and the added molecule. Cis-2,5-dimethyl-3-hexene undergoes addition reactions with a variety of reagents, including hydrogen, halogens, and water.
- Hydrogenation: When cis-2,5-dimethyl-3-hexene reacts with hydrogen in the presence of a catalyst, such as palladium or platinum, it undergoes hydrogenation, resulting in the formation of 2,5-dimethylhexane.
- Halogenation: Cis-2,5-dimethyl-3-hexene reacts with halogens, such as chlorine or bromine, to form vicinal dihalides. The addition of chlorine to cis-2,5-dimethyl-3-hexene yields 2,3-dichloro-2,5-dimethylhexane.
- Hydration: In the presence of water and an acid catalyst, cis-2,5-dimethyl-3-hexene undergoes hydration, resulting in the formation of 2,5-dimethyl-3-hexanol.
Substitution Reactions
In substitution reactions, one atom or group of atoms in the alkene is replaced by another atom or group of atoms. Cis-2,5-dimethyl-3-hexene undergoes substitution reactions with electrophilic reagents, such as hydrogen halides and sulfuric acid.
- Hydrohalogenation: Cis-2,5-dimethyl-3-hexene reacts with hydrogen halides, such as hydrogen chloride or hydrogen bromide, to form alkyl halides. The reaction with hydrogen chloride yields 2-chloro-2,5-dimethylhexane.
- Electrophilic Aromatic Substitution: Cis-2,5-dimethyl-3-hexene can also undergo electrophilic aromatic substitution reactions with aromatic compounds in the presence of an electrophile, such as a carbocation or a nitronium ion.
Elimination Reactions, Cis 2 5 dimethyl 3 hexene
In elimination reactions, a small molecule, such as water or hydrogen halide, is removed from the alkene, resulting in the formation of a new double bond or a triple bond. Cis-2,5-dimethyl-3-hexene undergoes elimination reactions in the presence of strong bases, such as sodium hydroxide or potassium tert-butoxide.
- Dehydration: When cis-2,5-dimethyl-3-hexene is heated in the presence of a strong base, it undergoes dehydration, resulting in the formation of 2,5-dimethyl-2-hexene.
- Dehydrohalogenation: Cis-2,5-dimethyl-3-hexene reacts with strong bases to undergo dehydrohalogenation, resulting in the formation of 2,5-dimethyl-3-hexyne.
Spectroscopic Analysis
Spectroscopic techniques provide valuable insights into the molecular structure and composition of cis-2,5-dimethyl-3-hexene. These techniques include infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and ultraviolet-visible (UV-Vis) spectroscopy.
Infrared (IR) Spectroscopy
IR spectroscopy measures the absorption of infrared radiation by a molecule, providing information about the functional groups present. In the IR spectrum of cis-2,5-dimethyl-3-hexene, the following peaks are observed:
- C-H stretching: 2960-2850 cm -1
- C=C stretching: 1640 cm -1
- C-H bending (alkene): 1450 cm -1
- C-H bending (methyl): 1375 cm -1
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy provides information about the hydrogen atoms in a molecule. The 1H NMR spectrum of cis-2,5-dimethyl-3-hexene shows the following peaks:
- H a(6H, singlet): 1.0 ppm (methyl groups)
- H b(2H, quartet): 1.6 ppm (CH 2group adjacent to the double bond)
- H c(2H, triplet): 2.1 ppm (CH 2group adjacent to the methyl groups)
- H d(1H, sextet): 5.4 ppm (CH group of the double bond)
Ultraviolet-Visible (UV-Vis) Spectroscopy
UV-Vis spectroscopy measures the absorption of ultraviolet and visible light by a molecule, providing information about the electronic structure. Cis-2,5-dimethyl-3-hexene absorbs UV light at a wavelength of 215 nm, which corresponds to the π → π* transition of the double bond.
Applications and Uses
Cis-2,5-dimethyl-3-hexene finds diverse applications in industries, primarily as a solvent, intermediate, and raw material.
Solvent
Due to its high solvency power, cis-2,5-dimethyl-3-hexene is employed as a solvent in various industries, including paints, coatings, and adhesives. Its ability to dissolve a wide range of organic compounds makes it a valuable component in formulations.
Intermediate
In the chemical industry, cis-2,5-dimethyl-3-hexene serves as an intermediate in the synthesis of other chemicals, such as fragrances, flavors, and pharmaceuticals. Its reactive nature allows for further functionalization and transformation into more complex molecules.
Raw Material
Cis-2,5-dimethyl-3-hexene is also used as a raw material in the production of synthetic polymers, such as polyisoprene and polybutadiene. These polymers are widely used in the manufacturing of tires, hoses, and other rubber products.
Safety and Handling
Cis-2,5-dimethyl-3-hexene, a flammable liquid, requires careful handling and storage to minimize potential hazards. This section provides comprehensive guidelines to ensure its safe use and prevent accidents.
Due to its volatility and low flash point, cis-2,5-dimethyl-3-hexene poses fire and explosion risks. Proper storage and handling practices are crucial to prevent incidents.
Storage
- Store in a cool, well-ventilated area away from heat, ignition sources, and incompatible materials.
- Keep containers tightly sealed to prevent evaporation and contamination.
- Store in a designated flammable liquid storage area that meets regulatory standards.
Handling
- Handle in a well-ventilated area or use appropriate personal protective equipment (PPE), such as gloves, goggles, and a respirator, to minimize exposure to vapors.
- Avoid contact with skin, eyes, and clothing. In case of contact, flush with plenty of water and seek medical attention if necessary.
- Use only non-sparking tools and equipment when handling cis-2,5-dimethyl-3-hexene.
Emergency Measures
- In case of a fire, use dry chemical, foam, or carbon dioxide extinguishers. Do not use water.
- Evacuate the area and call emergency services immediately.
- If a spill occurs, contain the liquid and absorb it with an inert material, such as sand or vermiculite. Dispose of the contaminated material properly.
Synthesis and Production
Cis-2,5-dimethyl-3-hexene can be synthesized through various methods, each involving distinct reaction mechanisms and conditions.
Alkylation of Alkenes
One common approach involves the alkylation of an alkene. In this process, an alkyl halide reacts with an alkene in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3). The reaction proceeds via an electrophilic addition mechanism, where the alkyl halide adds to the double bond of the alkene to form a carbocation.
This carbocation is then attacked by a nucleophile, such as a halide ion, to yield the desired product.
Wittig Reaction
Another method for synthesizing cis-2,5-dimethyl-3-hexene is the Wittig reaction. This reaction involves the reaction of an aldehyde or ketone with a phosphonium ylide. The phosphonium ylide is a nucleophilic species that attacks the carbonyl group of the aldehyde or ketone, resulting in the formation of an alkene.
The stereochemistry of the alkene product is determined by the configuration of the phosphonium ylide.
Hydroboration-Oxidation
Hydroboration-oxidation is a versatile method for the synthesis of alkenes. In this process, an alkene reacts with borane (BH3) to form a borane adduct. The borane adduct is then oxidized with hydrogen peroxide (H2O2) in the presence of a base, such as sodium hydroxide (NaOH), to yield the desired alkene.
The stereochemistry of the alkene product is determined by the regio- and stereoselectivity of the hydroboration step.
Comparison to Other Isomers
Cis-2,5-dimethyl-3-hexene is one of several isomers of dimethylhexene, each with its unique set of properties and reactivity. By comparing these isomers, we can gain insights into the effects of structural differences on their physical, chemical, and spectroscopic characteristics.
Physical Properties
- Boiling Point:The boiling point of cis-2,5-dimethyl-3-hexene (121-123 °C) is lower than that of its trans isomer (124-126 °C) due to weaker intermolecular forces in the cis form.
- Density:The density of cis-2,5-dimethyl-3-hexene (0.77 g/mL) is slightly lower than that of the trans isomer (0.78 g/mL).
- Refractive Index:The refractive index of cis-2,5-dimethyl-3-hexene (1.434) is similar to that of the trans isomer (1.436).
Chemical Reactivity
- Electrophilic Addition:Both cis- and trans-2,5-dimethyl-3-hexene undergo electrophilic addition reactions, but the cis isomer reacts more slowly due to steric hindrance.
- Polymerization:The cis isomer is more likely to polymerize than the trans isomer due to its more reactive double bond.
Spectroscopic Characteristics
- NMR Spectroscopy:The NMR spectra of cis- and trans-2,5-dimethyl-3-hexene show distinct differences in the chemical shifts of the protons on the double bond.
- IR Spectroscopy:The IR spectra of the two isomers show different absorption bands corresponding to the stretching vibrations of the C=C double bond.
Detailed FAQs
What is the molecular formula of cis-2,5-dimethyl-3-hexene?
C8H16
What is the boiling point of cis-2,5-dimethyl-3-hexene?
121-123 °C
What is the density of cis-2,5-dimethyl-3-hexene?
0.73 g/cm³
What is the refractive index of cis-2,5-dimethyl-3-hexene?
1.420