Express the concentration of a 0.0570 m – Embark on a journey into the realm of molarity, where we delve into the intricacies of expressing the concentration of a 0.0570 M solution. This comprehensive guide will illuminate the concepts, calculations, and applications surrounding this fundamental aspect of chemistry, providing a robust foundation for understanding and manipulating solutions.
The precise definition of molarity and its formula lay the groundwork for our exploration. We will unravel the relationship between molarity and volume, empowering you to determine the number of moles of solute present in a given solution. The specific concentration of 0.0570 M takes center stage, as we explore the steps involved in preparing a solution with this exact concentration.
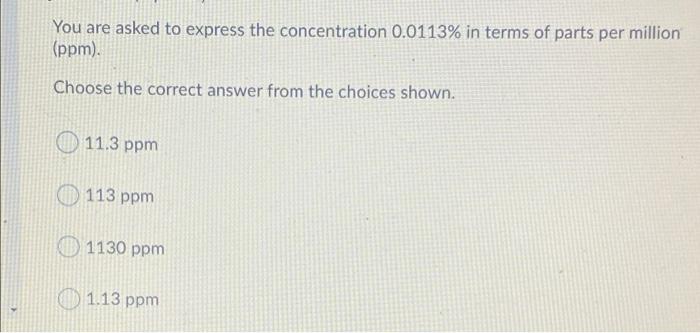
Expressing Concentration
Molarity (M) is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. The formula for molarity is:
M = moles of solute / liters of solution
To calculate the number of moles of solute in a given volume of solution, multiply the molarity by the volume in liters:
moles of solute = M x liters of solution
The relationship between molarity and volume is that the molarity of a solution is inversely proportional to its volume. This means that if you increase the volume of a solution without adding more solute, the molarity will decrease.
Specific Concentration: 0.0570 M, Express the concentration of a 0.0570 m
A 0.0570 M solution contains 0.0570 moles of solute per liter of solution. To prepare a 0.0570 M solution, you need to dissolve 0.0570 moles of solute in enough solvent to make 1 liter of solution.
The steps involved in preparing a 0.0570 M solution are as follows:
- Calculate the mass of solute needed using the molar mass of the solute.
- Dissolve the calculated mass of solute in a small amount of solvent.
- Transfer the dissolved solute to a volumetric flask.
- Add solvent to the volumetric flask until the solution reaches the calibration mark.
Units and Notation
Molarity is expressed in units of moles per liter (M). The proper notation for molarity is to use the uppercase letter M, without a period.
0.0570 M can be converted to other units of concentration using the following conversion factors:
- 1 M = 1000 mM
- 1 M = 1 mol/kg solvent
- 1 M = 1000 ppm
Applications of Molarity
Molarity is commonly used in scientific and industrial settings to express the concentration of solutions. Some examples of where molarity is used include:
- Preparing solutions for chemical reactions
- Analyzing the concentration of solutions in environmental samples
- Determining the amount of substance present in a solution
Accurate molarity determination is important in various applications, such as:
- Ensuring the correct concentration of solutions for chemical reactions
- Providing accurate data for environmental analysis
- Calculating the amount of substance present in a solution for research or industrial purposes
Dilution and Concentration
Dilution is the process of adding solvent to a solution to decrease its concentration. The formula for calculating the new concentration after dilution is:
M1V1 = M2V2
where:
- M1 is the initial molarity
- V1 is the initial volume
- M2 is the final molarity
- V2 is the final volume
For example, to dilute a 0.0570 M solution to a concentration of 0.0285 M, you would add enough solvent to increase the volume of the solution to 2 liters.
Popular Questions: Express The Concentration Of A 0.0570 M
What is the formula for molarity?
Molarity (M) = moles of solute / liters of solution
How do I calculate the number of moles of solute in a solution?
Moles of solute = Molarity (M) x Volume of solution (L)
What is the relationship between molarity and volume?
Molarity is inversely proportional to volume. As volume increases, molarity decreases, and vice versa.



