Embark on an enlightening journey with the Introduction to Balancing Equations Worksheet, a meticulously crafted guide that unveils the intricate art of chemical equation balancing. This worksheet serves as a cornerstone for comprehending the fundamental principles of chemistry, empowering students with the knowledge and skills to navigate complex chemical reactions with precision and confidence.
Delving into the realm of balanced equations, this worksheet provides a comprehensive exploration of their significance in chemistry. Through real-world examples and interactive exercises, students gain a deep understanding of the crucial role equations play in predicting the outcome of chemical reactions and unraveling the mysteries of the molecular world.
Introduction to Balancing Equations
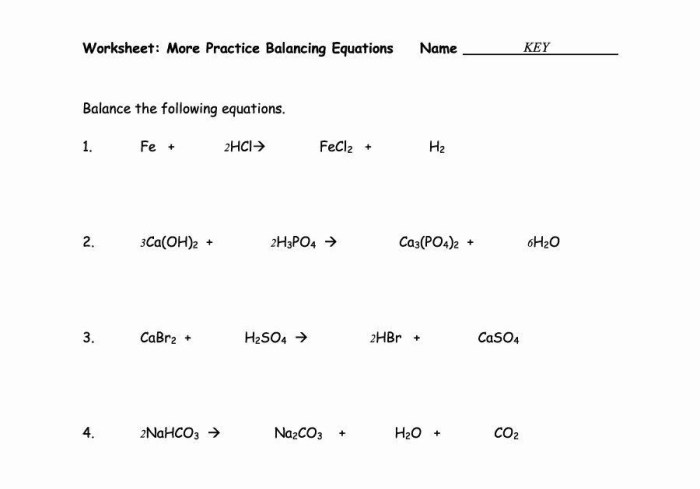
Balancing chemical equations is a crucial skill in chemistry, ensuring that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
In the real world, balanced equations play a vital role in various applications, such as predicting the stoichiometry of reactions in industrial processes, designing experiments, and understanding the mechanisms of chemical reactions.
Methods for Balancing Equations, Introduction to balancing equations worksheet
There are several methods for balancing equations, including the step-by-step method and the half-reaction method. The step-by-step method involves adjusting the stoichiometric coefficients of the reactants and products until the equation is balanced for each element.
The half-reaction method is used for balancing redox reactions, where oxidation and reduction occur simultaneously. In this method, the reaction is divided into two half-reactions, one for oxidation and one for reduction, which are then balanced separately before combining them to obtain the overall balanced equation.
Practice Problems and Solutions
Practice problems are essential for developing proficiency in balancing equations. These problems can range from simple to complex, covering a variety of reaction types.
Detailed solutions to these problems should be provided, explaining the steps involved in balancing the equation and highlighting any specific techniques or concepts used.
Advanced Concepts in Balancing Equations
Beyond the basic techniques, there are advanced concepts in balancing equations that are important for understanding more complex chemical reactions.
These concepts include the identification of limiting reactants and excess reactants, which determine the stoichiometry of the reaction. Balancing equations in solution, including precipitation reactions, is also crucial for understanding reactions in aqueous environments.
Additionally, complex balancing equations involving multiple reactions require a systematic approach to ensure that all reactants and products are accounted for.
FAQ Guide: Introduction To Balancing Equations Worksheet
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation equals the number of atoms of that element on the products’ side. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Why is it important to balance equations in chemistry?
Balancing equations is crucial in chemistry because it allows us to determine the exact amounts of reactants and products involved in a chemical reaction. This information is essential for predicting the outcome of reactions, designing experiments, and understanding the stoichiometry of chemical processes.

