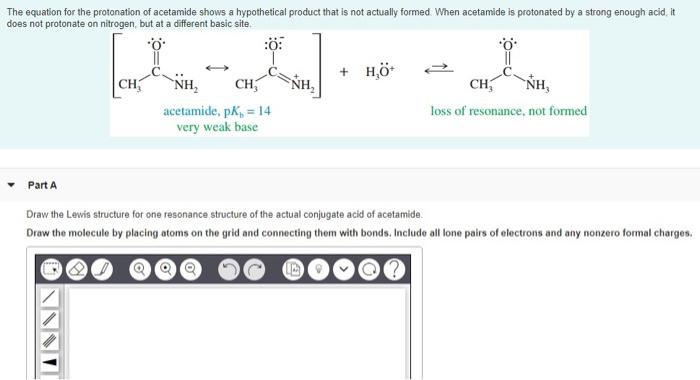
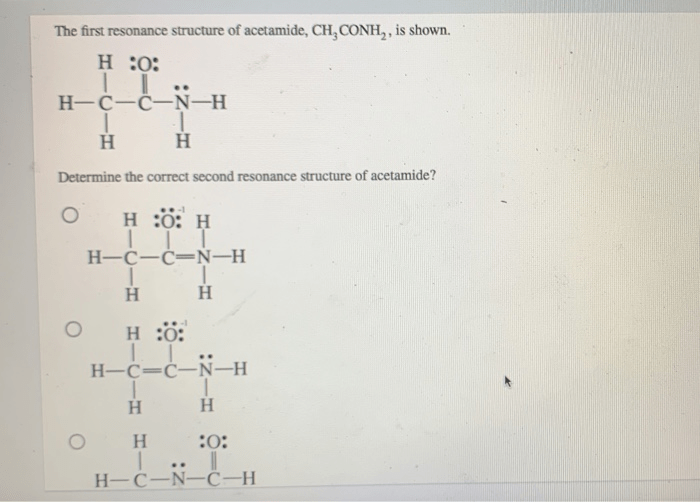
Draw the lewis structures for resonance forms of acetamide – The concept of resonance plays a crucial role in understanding the electronic structure and properties of organic molecules. In this context, we will delve into the resonance forms of acetamide, exploring their structural features, physical and chemical properties, and diverse applications.
Acetamide, an important amide derivative, exhibits resonance due to the delocalization of electrons within its molecular structure. This phenomenon gives rise to multiple resonance forms, each contributing to the overall electronic distribution and influencing the molecule’s behavior.
Resonance Forms of Acetamide
Acetamide is an organic compound with the chemical formula CH 3CONH 2. It is a white, crystalline solid that is soluble in water. Acetamide is a versatile compound that is used in a variety of applications, including as a solvent, a plasticizer, and a precursor to other chemicals.
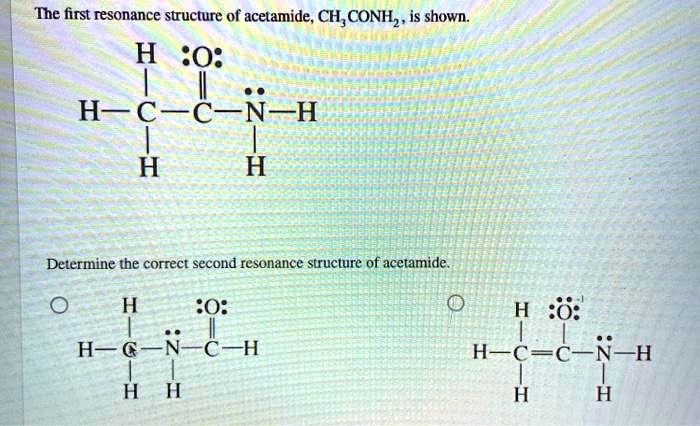
Acetamide exists in two resonance forms, which are shown below:
- Form A:In this form, the double bond is between the carbon and oxygen atoms, and the nitrogen atom has a lone pair of electrons.
- Form B:In this form, the double bond is between the carbon and nitrogen atoms, and the oxygen atom has a lone pair of electrons.
The two resonance forms of acetamide are in equilibrium with each other, and the actual structure of acetamide is a hybrid of the two forms.
Structural Features of Acetamide
Acetamide contains two functional groups: an amide group and an amino group. The amide group is a functional group that consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a nitrogen atom. The amino group is a functional group that consists of a nitrogen atom bonded to two hydrogen atoms.
The carbon atom in the amide group is sp 2hybridized, and the nitrogen atom in the amide group is sp 2hybridized. The nitrogen atom in the amino group is sp 3hybridized.
The resonance forms of acetamide affect the bond lengths and angles in the molecule. In Form A, the C-O bond is shorter than the C-N bond, and the N-H bonds are shorter than the C-H bonds. In Form B, the C-N bond is shorter than the C-O bond, and the C-H bonds are shorter than the N-H bonds.
Physical and Chemical Properties
Acetamide is a white, crystalline solid with a melting point of 82 °C and a boiling point of 222 °C. It is soluble in water, alcohol, and ether.
Acetamide is a relatively unreactive compound. It does not react with strong acids or bases, and it is not oxidized by air. However, acetamide does react with some reducing agents, such as sodium borohydride.
Acetamide undergoes a variety of reactions, including hydrolysis, acylation, and alkylation.
Applications of Acetamide: Draw The Lewis Structures For Resonance Forms Of Acetamide
Acetamide is used in a variety of applications, including:
- As a solvent:Acetamide is used as a solvent for a variety of organic compounds, including dyes, oils, and waxes.
- As a plasticizer:Acetamide is used as a plasticizer for a variety of polymers, including polyvinyl chloride (PVC) and polyvinyl acetate (PVAc).
- As a precursor to other chemicals:Acetamide is used as a precursor to a variety of other chemicals, including acetic acid, acetamide, and N-methylacetamide.
Essential FAQs
What is resonance?
Resonance is a concept that describes the delocalization of electrons within a molecule, resulting in multiple contributing structures that represent the overall electronic distribution.
How many resonance forms does acetamide have?
Acetamide has two primary resonance forms, which differ in the placement of the double bond and the charge distribution.
What is the hybridization of the carbon atom in the amide bond of acetamide?
The carbon atom in the amide bond of acetamide is sp 2hybridized.