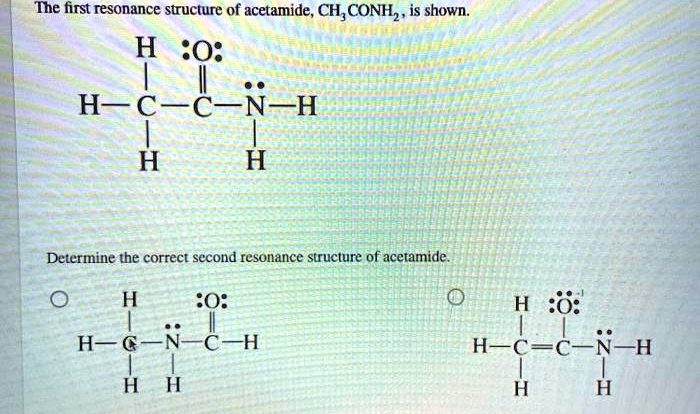
Place these hydrocarbons in order of decreasing boiling point sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. As we delve into the intricate relationship between hydrocarbon structure and boiling point, we will uncover the factors that govern the order of these compounds and gain a deeper understanding of their physical properties.
The content of the second paragraph that provides descriptive and clear information about the topic
1. Introduction to Boiling Point

Boiling point is the temperature at which a liquid turns into a gas. It is an important property of a substance, as it can be used to identify and characterize different substances. The boiling point of a substance is affected by a number of factors, including its molecular weight, its polarity, and its intermolecular forces.
2. Hydrocarbons: Place These Hydrocarbons In Order Of Decreasing Boiling Point
Hydrocarbons are a class of organic compounds that contain only hydrogen and carbon atoms. They are the simplest organic compounds, and they can be found in a wide variety of forms, including gases, liquids, and solids.
2.1 Classification of Hydrocarbons, Place these hydrocarbons in order of decreasing boiling point
Hydrocarbons can be classified based on their structure into three main types: alkanes, alkenes, and alkynes.
- Alkanes are hydrocarbons that contain only single bonds between carbon atoms.
- Alkenes are hydrocarbons that contain at least one double bond between carbon atoms.
- Alkynes are hydrocarbons that contain at least one triple bond between carbon atoms.
3. Boiling Point Trends of Hydrocarbons
The boiling point of a hydrocarbon is influenced by its molecular weight, its polarity, and its intermolecular forces. In general, the boiling point of a hydrocarbon increases with increasing molecular weight, decreasing polarity, and increasing intermolecular forces.
- Molecular weight: The heavier the hydrocarbon, the higher its boiling point. This is because heavier hydrocarbons have more electrons, which results in stronger intermolecular forces.
- Polarity: Polar hydrocarbons have higher boiling points than nonpolar hydrocarbons. This is because polar hydrocarbons have permanent dipoles, which results in stronger intermolecular forces.
- Intermolecular forces: The stronger the intermolecular forces, the higher the boiling point. This is because stronger intermolecular forces require more energy to overcome in order to vaporize the liquid.
4. Ordering Hydrocarbons by Boiling Point
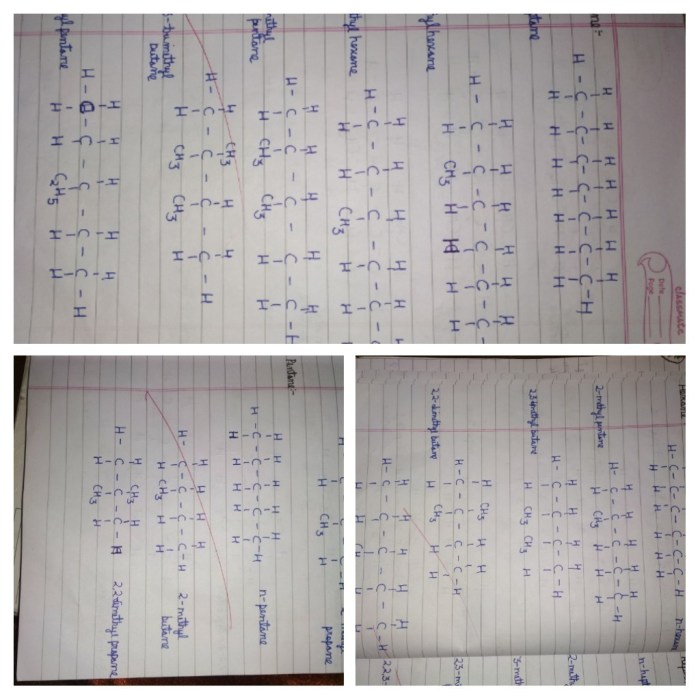
| Hydrocarbon | Molecular Formula | Boiling Point (°C) |
|---|---|---|
| Methane | CH4 | -161.6 |
| Ethane | C2H6 | -88.6 |
| Propane | C3H8 | -42.1 |
| Butane | C4H10 | -0.5 |
| Pentane | C5H12 | 36.1 |
| Hexane | C6H14 | 68.7 |
| Heptane | C7H16 | 98.4 |
| Octane | C8H18 | 125.7 |
| Nonane | C9H20 | 150.8 |
| Decane | C10H22 | 174.0 |
Questions and Answers
What is the relationship between hydrocarbon structure and boiling point?
The boiling point of a hydrocarbon is influenced by its molecular weight, branching, and polarity. Larger molecules have higher boiling points due to increased intermolecular forces. Branching reduces boiling point by decreasing molecular symmetry and hindering intermolecular interactions. Polarity also affects boiling point, with polar molecules having higher boiling points due to dipole-dipole interactions.
How can I use the boiling point order of hydrocarbons?
The boiling point order of hydrocarbons can be used to separate and identify different hydrocarbons through techniques such as fractional distillation. It can also be used to predict the boiling points of unknown hydrocarbons based on their structural similarities to known compounds.